The
fibrinolytic system dissolves fibrin blood clots, acting in reverse to the
coagulation system.
▼
ε-aminocaproic acid :
antifibrinolytics :
fibrin clots :
fibrin degradation products :
fibrinolysis :
kringle domains :
PAI-1,
PAI-2 :
plasmin :
plasminogen :
plasminogen activator inhibitors :
serine proteases :
serpins :
thrombolytic agents :
tissue plasminogen activator (
PLAT,
tPA) :
tranexamic acid :
uPA :
urokinase :
urokinase receptor :
zymogen ▼
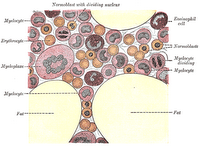
Plasminogen, the inactive,
zymogen form of
plasmin, is incorporated into
fibrin clots as they form. Cleavage of plasminogen by
tissue plasminogen activator (PLAT, tPA) and
urokinase converts plasminogen to the active
serine protease plasmin form. Acting as a serine protease, the
kringle domains of
plasmin bind to
arginine and
lysine residues and cleave fibrin into
fibrin degradation products (
fibrinolysis).
Tissue plasminogen activator (PLAT, tPA) is a
serine protease secreted by cells of the arteriolar endothelium.
Urokinase, also termed
urokinase-type plasminogen activator (uPA), is also a serine protease that contains a
serine protease domain, a
kringle domain, and a
growth factor domain. The
serpins, plasminogen activator inhibitor-1 (
PAI-1) and plasminogen activator inhibitor-2 (
PAI-2) irreversibly inhibit the
protease (
peptidase) activity of
tPA and
uPA.
In the
extracellular matrix,
urokinase binds to the
urokinase receptor, tethering
urokinase to the
cell membrane. Through its interaction with the urokinase receptor, urokinase participates in cell
adhesion,
migration, and cellular
mitotic pathways. It appears that tissue degradation following plasminogen activation facilitates tissue invasion and contributes to establishment of
tumor metastasis, making
urokinase an attractive potential target for anticancer inhibitors.
Urokinase is employed as a
thrombolytic agent in the treatment of deep venous thrombosis (DVT) and pulmonary embolism (PE). Both
urokinase and recombinant
tissue plasminogen activator (PLAT, tPA) are employed in treatment of myocardial infarction (MI), and recombinant
PLAT is used in treatment of acute stroke (CVA, cerebrovascular accident).
Conversely,
antifibrinolytics, such as
aminocaproic acid (ε-aminocaproic acid) and the more potent
tranexamic acid are employed as
inhibitors of
fibrinolysis. They act by blocking the
lysine-binding site in the
kringle domains on plasminogen, and are employed in treatment of menorrhagia, excessive post-operative bleeding, and bleeding dyscrasias.
▲
Top ▲
tags
[Tissue] [fibrinolysis] [metastasis] [DVT] [PE] [MI] [CVA] [thrombolytic agent] [antifibrinolytics]
|


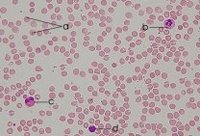 Blood is a highly specialized tissue produced in the bone marrow in a process called hematopoiesis. Blood contains red blood cells (a=erythrocytes) and white cells (leukocytes, b=neutrophil, c=eosinophil, d=lymphocyte) circulating in plasma accompanied by platelets, plasma proteins, and other dissolved substances.
Blood is a highly specialized tissue produced in the bone marrow in a process called hematopoiesis. Blood contains red blood cells (a=erythrocytes) and white cells (leukocytes, b=neutrophil, c=eosinophil, d=lymphocyte) circulating in plasma accompanied by platelets, plasma proteins, and other dissolved substances.